NAFDAC Raises Alarm Over Surge of Smuggled Prohibited Food Products in Nigerian Markets
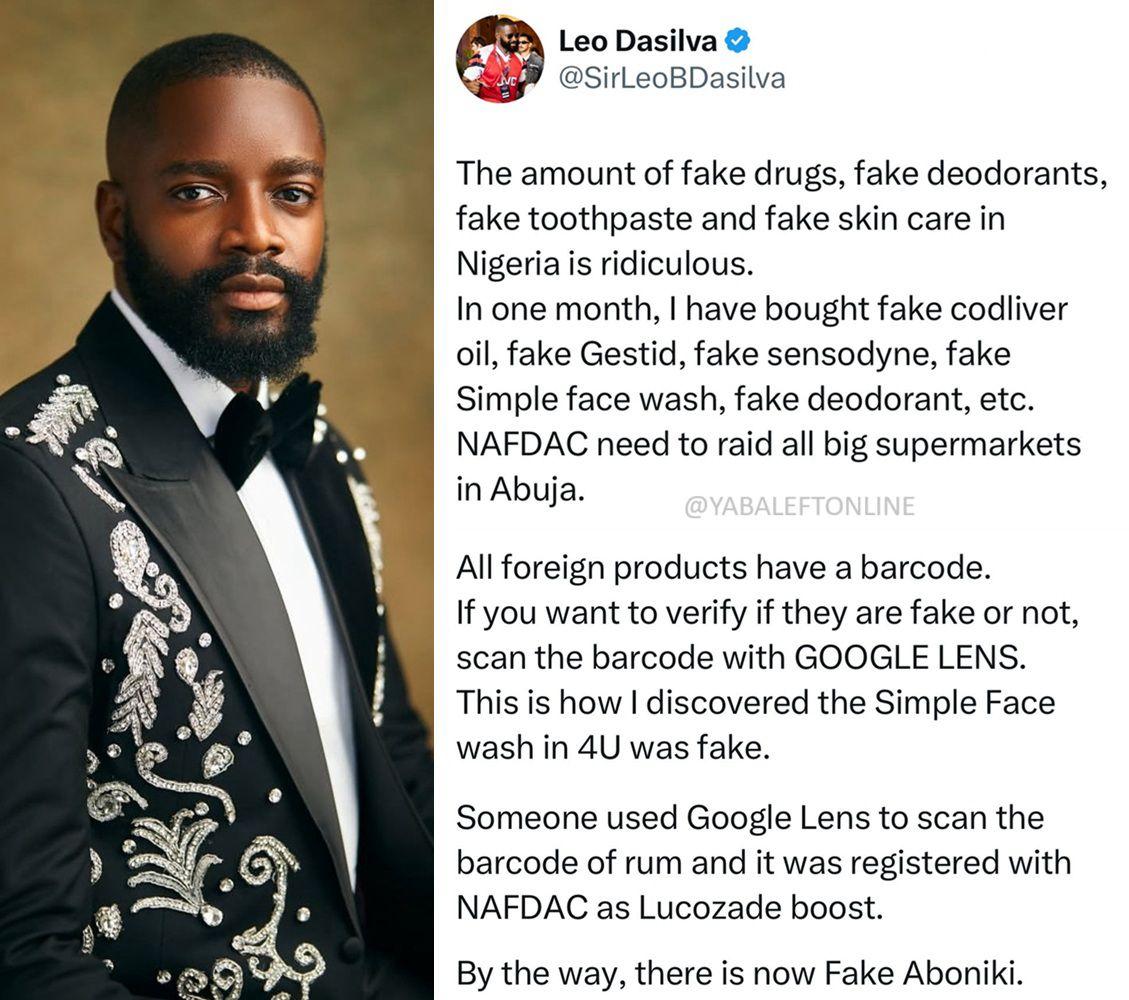
NAFDAC has issued a public warning about the increasing circulation of prohibited food products—including pasta, noodles, sugar, and tomato paste—illegally smuggled into Nigerian markets. The agency says these items, listed on the Customs Prohibition List, bypass mandatory safety checks and pose serious health risks. NAFDAC has ordered importers, supermarkets, and traders to immediately stop the sale and distribution of such products or face seizures, licence revocations, and prosecution. The agency also called for coordinated action with Customs, Immigration, SON, NPA, NIMASA, NAQS, and other regulators to prevent further influx of unsafe items. NAFDAC reaffirmed its commitment to protecting consumers and enforcing food safety regulations nationwide.
NAFDAC has issued a public warning about the increasing circulation of prohibited food products—including pasta, noodles, sugar, and tomato paste—illegally smuggled into Nigerian markets. The agency says these items, listed on the Customs Prohibition List, bypass mandatory safety checks and pose serious health risks. NAFDAC has ordered importers, supermarkets, and traders to immediately stop the sale and distribution of such products or face seizures, licence revocations, and prosecution. The agency also called for coordinated action with Customs, Immigration, SON, NPA, NIMASA, NAQS, and other regulators to prevent further influx of unsafe items. NAFDAC reaffirmed its commitment to protecting consumers and enforcing food safety regulations nationwide.
NAFDAC Raises Alarm Over Surge of Smuggled Prohibited Food Products in Nigerian Markets
NAFDAC has issued a public warning about the increasing circulation of prohibited food products—including pasta, noodles, sugar, and tomato paste—illegally smuggled into Nigerian markets. The agency says these items, listed on the Customs Prohibition List, bypass mandatory safety checks and pose serious health risks. NAFDAC has ordered importers, supermarkets, and traders to immediately stop the sale and distribution of such products or face seizures, licence revocations, and prosecution. The agency also called for coordinated action with Customs, Immigration, SON, NPA, NIMASA, NAQS, and other regulators to prevent further influx of unsafe items. NAFDAC reaffirmed its commitment to protecting consumers and enforcing food safety regulations nationwide.
0 Comentários
·0 Compartilhamentos
·218 Visualizações